
Accreditations
FDA Accreditation Scheme for Conformity Assessment (ASCA)
What is FDA Accreditation Scheme for Conformity Assessment (ASCA)?
ANAB provides accreditation for the U.S. Food and Drug Administration Accreditation Scheme for Conformity Assessment (ASCA) program as an FDA-recognized accreditation body.
The voluntary ASCA program is intended to increase consistency and predictability in the FDA’s approach to assessing conformance with FDA-recognized consensus standards and test methods. Thus, the ASCA program should enhance product reviewers’ and device manufacturers’ confidence in medical device testing.
This, in turn, is intended to decrease the need for the FDA to request additional information about testing methodologies when a premarket submission includes declarations of conformity to an FDA-recognized consensus standard eligible for inclusion in the ASCA program.
Ultimately, the program is designed to help ensure patients have timely access to safe, effective, and high-quality medical devices.
The ASCA program relies on international conformity assessment standards and a set of FDA-identified ASCA program specifications. The program includes both cross-cutting and device-specific standards. The FDA selected standards and tests from the biocompatibility and basic safety and essential performance series. The standards have public health significance and have or are able to provide the means for establishing acceptance criteria.
Access FDA ASCA Documents and Information
If you’re already accredited to ISO/IEC 17025, you may be able to become accredited for the ASCA program via scope extension.
Steps to ASCA Accreditation
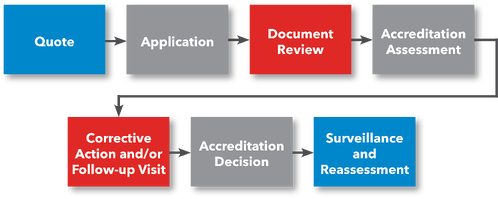
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
- Laboratory uses ANAB Accreditation and Submits Application to FDA for FDA Approval
Talk to an Expert
Fitri Sudradjat
Manager of Accreditation, Inspection, Laboratories, and Related Activities
414-501-5451

Randy Long
Senior Manager of Accreditation,
Inspection, Laboratories, and Related Activities
414-501-5339

Need Training To Support Your Accreditation Journey?
Learn at your own pace with online courses or choose an instructor led class offered online or in a convenient location.


