
Accreditations
ISO/IEC 17025 Cannabis Testing Laboratory Accreditation
What is ISO/IEC 17025 Cannabis Testing Laboratory Accreditation?
ANAB’s testing laboratories performing chemical, microbiological, and/or non-destructive analyses in the examination or sampling of (but not limited to) cannabis and cannabis-derived products, cannabis ingredients in the production of food, in-process cannabis samples, and final products fall under the ISO/IEC 17025 Cannabis Testing Laboratory Accreditation Program.
In addition to the Cannabis Testing Laboratory Accreditation Program, the Michigan Marijuana Regulatory Agency (MRA) Marijuana Sampling and Testing Accreditation Program supplemental program (SR 2436) is applicable to Cannabis Testing Laboratories operating in the State of Michigan.
Steps to ISO/IEC 17025 Cannabis Testing Laboratory Accreditation
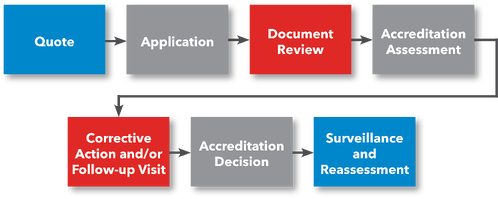
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
Supplemental Program Requirements
SR 2436: Michigan Marijuana Regulatory Agency (MRA)Marijuana Sampling and Testing Accreditation Program
This program is designed for Cannabis Testing Laboratories operating in the State of Michigan.
Talk to an Expert
Chris Fox-Strauss
Manager of Accreditation, Inspection, Laboratories, and Related Activities
414-501-5448

Need Training To Support Your Accreditation Journey?
Learn how to implement an accreditation program. Register for a course to get in-depth instruction on accreditation-related requirements and processes.


