
Accreditations
FDA Laboratory Accreditation for Analyses of Foods (LAAF) Program
What is the FDA Laboratory Accreditation for Analyses of Foods (LAAF) Program?
ANAB accredits food testing laboratories under the US Food and Drug Administration (FDA) Laboratory Accreditation for Analyses of Foods (LAAF Program).
about the fda laaf program
The FDA established the Laboratory Accreditation for Analyses of Foods (LAAF) program as required by FSMA section 202(a), which added section 422 to the Federal Food, Drug, and Cosmetic (FD&C) Act (21 U.S.C. 350k). The FDA Food Safety Modernization Act (FSMA) final rule on Laboratory Accreditation for Analyses of Foods (LAAF) sets a laboratory accreditation program for the testing of food in certain circumstances.
The use of LAAF-accredited laboratories is required, for example, when addressing an identified or suspected food safety program, to support removal of a food from an import alert, in support of admission of an article of food, or as required by a directed food laboratory order. Foods included in the program are bottled water, shell eggs, sprouts, and import/export food products, and foods suspected of adulteration.
about laaf accreditation
Effective June 1, 2022, ANAB has been accepted as a recognized accreditation body (AB) for the FSMA LAAF program, meeting Accreditation Body eligibility requirements and demonstrating sufficient Accreditation Body Capacity to accredit labs to the standards established under the final rule. ANAB-specific supplemental requirements have been implemented for the LAAF program. Laboratories participating in the voluntary LAAF program are also accredited to ISO/IEC 17025:2017 for test methods included in the LAAF program.
FSMA Final Rule on Laboratory Accreditation for Analyses of Foods (LAAF)
Steps to LAAF Accreditation
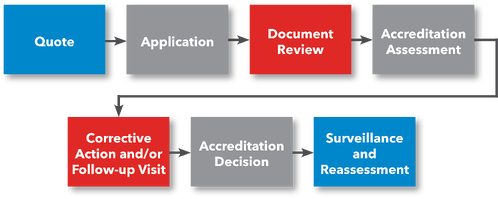
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
Supplemental Program Requirements
Talk to an Expert
Fitri Sudradjat
Manager of Accreditation, Inspection, Laboratories, and Related Activities
414-501-5451

Need Training To Support Your Accreditation Journey?
Learn how to implement an accreditation program. Register for a course to get in-depth instruction on accreditation-related requirements and processes. Learn at your own pace with online courses or choose an instructor led class offered online or in a convenient location.


