
Accreditations
ISO 20387 Biobanking Accreditation
What is ISO 20387 Biobanking Accreditation?
The ANAB biobanking accreditation program was designed to improve the quality of biobanks through assuring compliance with the international standard, ISO 20387. ANAB accreditation will help move your organization toward global recognition, consistent operations, and a competitive advantage and will provide assurance of the quality of biobanking activities with the objective of promoting confidence in biobanking.
Biobanking accreditation is applicable to all organizations performing biobanking, including biobanking of biological material from multicellular organisms (e.g. human, animal, fungus and plant) and microorganisms for research and development. These can involve different types of organizations from independent legal entities or may reside within governmental entities, academic institutions, hospitals, non-profit or commercial organizations.
Biobanks may vary in the types of activities they perform. They can either perform the full range of activities including collecting/acquisitioning, preparing, preserving, testing, storage, analyzing and distributing biological materials and data, or a subset of these activities.
The ANAB biobanking accreditation program was designed to assure the quality of biobanks and the data produced through assuring compliance with ISO 20387. The objective of ISO 20387 is to promote confidence in biobanking. It contains requirements to enable biobanks to demonstrate competent biobank operation and the ability to provide biological material and associated data of appropriate quality for research and development.
Our goal through biobanking accreditation is to improve the quality of biobanks and the quality of the data they produce. ANAB accreditation managers and biobank experts have practical experience in the field and are often viewed as peers to the biobank.
The ANAB biobanking accreditation program is well-established and should be your choice for accreditation of biobanking activities.
Based on ISO 20387, General requirements for biobanking, and ISO 9001, Quality management systems – Requirements, ANAB’s ISO 20387 program includes four additional criteria for accredited organizations:
- Maintain a quality control plan which includes an evaluation of risk and is retained for a minimum of 4 years.
- Promptly notify ANAB of unsatisfactory results.
- Perform and document appropriate corrective action if ISO 20387 non-conformities are discovered.
- Prove metrological traceability (MT) to the International System of Units (SI).
steps to ISO 20387 Biobanking Accreditation
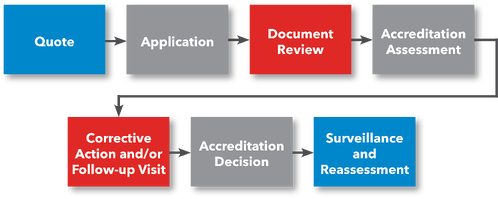
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
ISO 20387 accreditation involves an independent assessment of the biobanking organization that includes an examination of the quality management system, personnel qualifications and competence, material handling, storage environment and equipment, and reporting. Qualified assessors conduct a thorough evaluation of all factors affecting the storage of biological material and the demographic sample information.
To ensure continued compliance, accredited biobank organizations are regularly re-assessed to ensure they maintain their technical expertise and competence. Accredited organizations are also required to participate in proficiency testing programs (EQAs).
Featured Products
ISO 20387:2018
Biotechnology – Biobanking – General Requirements For Biobanking
This document specifies general requirements for the competence, impartiality and consistent operation of biobanks including quality control requirements to ensure biological material and data collections of appropriate quality.
ISO TR 227582 Biotechnology – Biobanking – Implementation Guide For ISO 20387
This document provides guidance to biobanks on how to implement the quality management, management, and technical requirements of ISO 20387.
ASK AN EXPERT
Fitri Sudradjat
Manager of Accreditation,
Inspection, Laboratories, and Related Activities
414-501-5451

Need Training To Support Your Accreditation Journey?
Learn how to implement an accreditation program. Register for a course to get in-depth instruction on accreditation-related requirements and processes. Learn at your own pace with online courses or choose an instructor led class offered online or in a convenient location..


