
Accreditations
Food and Pharmaceutical Reference Material Producer (RMP) Accreditation
What is Food and Pharmaceutical Reference Material Producer (RMP) Accreditation?
The ANAB Reference Material Producer (RMP) accreditation program is designed for organizations seeking to produce any type of food and pharmaceutical related certified reference materials (CRM) and/or reference materials (RM).
ANAB RMP accreditation program will help assure the quality of certified reference materials (CRM) and reference materials (RM) used for food and pharmaceutical testing by assuring compliance to the international standard ISO 17034 and ANAB accreditation requirements based on national and international requirements.
Reference Material Producers must follow criteria designed to assure the quality of CRMs and RMs production. This includes requirements for RMP operations and for demonstrating competence in the planning, control, production, assignment of property values, and distribution of CRMs and RMs.
RMPs can be accredited to produce a wide variety of food and pharmaceutical industry related CRMs and RMs used for critical and measurable properties along with establishing metrological traceability. Certified reference materials (CRM) and reference materials (RM) are developed to specific levels of accuracy so that reference material can be individually selected to assure it is appropriate for its intended use.
Our goal through RMP accreditation is to improve the quality of RMPs and the quality of the certified reference materials (CRM) and reference materials (RM) they produce. ANAB accreditation managers and RMP experts have practical experience in the field and are often viewed as peers to the RMP.
The ANAB RMP accreditation program is well-established and should be your choice for accreditation for organizations perform sample collection activities.
Steps to Food and Pharmaceutical RMP ACCREDITATION
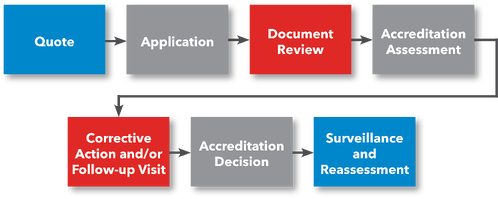
Steps for Getting Accredited
- Request a Quote
- File Application
- Prepare for Accreditation Assessment
- Submit Documentation for Review
- (Optional) Preliminary Assessment
- Accreditation Assessment
- Corrective Action (if applicable)
- ANAB Accreditation Decision
- Receive Accreditation Certificate
ISO 17034
General Requirements for the Competence of Reference Material Producers
ISO 17034 specifies general requirements for the competence and consistent operation of reference material producers.
ISO 17034 sets out the requirements in accordance with which reference materials are produced. It is intended to be used as part of the general quality assurance procedures of the reference material producer.
ISO 17034 covers the production of all reference materials, including certified reference materials.
Talk to an Expert
Patrick Selig
Senior Manager of Accreditation, Inspection, Laboratories, and Related Activities
414-501-5475

Need Training To Support Your Accreditation Journey?
Learn how to implement an accreditation program. Register for a course to get in-depth instruction on accreditation-related requirements and processes.

